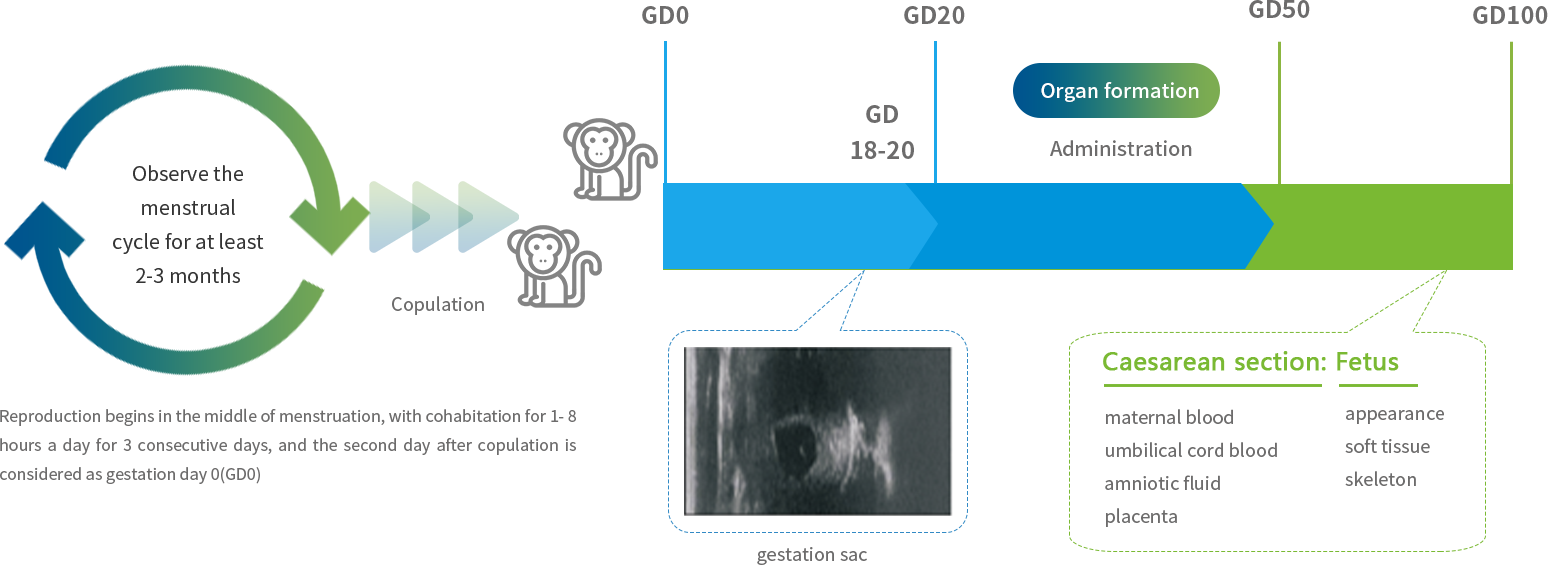
Nonclinical Safety Evaluation
Topgenebio provides GLP-compliant preclinical safety evaluation services for chemical drugs, traditional Chinese medicine, and biotechnological drugs, meeting both domestic and international regulatory requirements for submission.
Area: Over 24000㎡
Project Experience:
Over 1200 specialized studies
Over 200 varieties of new drug declarations
Over 60 domestic and international declaration projects







 Service Capabilities
Service Capabilities